Roflumilast - Intermediate
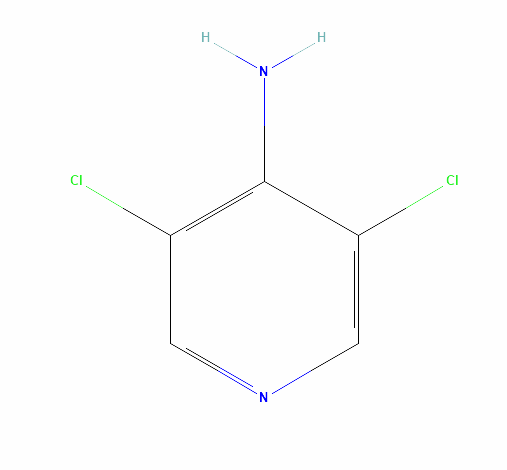
Name of Intermediate: 3,5-Dichloro-4-Amino Pyridine
Name of API: Roflumilast
CAS No: 22889-78-7
Our Roflumilast intermediates are developed to meet stringent global standards for Active Pharmaceutical Ingredients (APIs). Manufactured using advanced synthesis routes and precise quality control, they enable efficient, high-yield production of Roflumilast. With scalable and reproducible processes, we ensure consistent purity and stability from lab-scale development to commercial manufacturing. Each intermediate is optimized for process efficiency, regulatory compliance, and reliable performance in formulation. Partnering with SSPharma gives you access to high-quality Roflumilast intermediates that support faster development cycles, robust supply chains, and seamless transition from R&D to large-scale production — empowering innovation in diabetes therapeutics.

Name of Intermediate: 3,5-Dichloro-4-Amino Pyridine
Name of API: Roflumilast
CAS No: 22889-78-7