API INTERMEDIATES
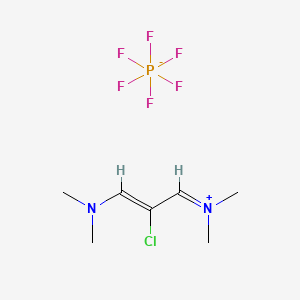
2-Chloro-1,3-bis(dimentylamino)trimethinium hexafluorophosphate (CAS No.: 249561-98-6)
SS Pharma Group is an ISO 9001:2015 Certified Global Supplier based in India for the product CAS No.: 249561-98-6. We supply this product from our network of trusted manufacturers, suppliers, and principals, catering to your Research and Development, Evaluation, or Commercial requirements based on product availability. We can also provide upstream or downstream intermediates along with a supportive technical package for evaluation upon request.
We respect our customers' stringent regulatory and specification needs, ensuring transparency in offering intermediates. SS Pharma Group prioritizes affordability and high-quality raw material intermediates, making it easy to access reliable manufacturers, suppliers, and principals through our extensive network.
If you are seeking upstream or downstream intermediates related to CAS No.: 249561-98-6, please feel free to email us. Based on your specific requirements, we will source a reliable manufacturer, supplier, or principal that meets your quality standards and aligns with your needs.
CAS number:
249561-98-6
Chemical Name:
N,N-DiMethyl-2-chloro-triMethiniuM Hexafluorophosphate, 2-Chloro-1,3-bis(dimentylamino)trimethinium hexafluorophosphate (CAS No.: 249561-98-6)
Manufacturer Certificate:
GMP, Factory GMP, ISO Certified
| Synonyms | N,N-DiMethyl-2-chloro-triMethiniuM Hexafluorophosphate, 2-Chloro-1,3-bis(dimentylamino)trimethinium hexafluorophosphate (CAS No.: 249561-98-6) |
|---|---|
| Molecular Formula | C7H14ClN2.PF6 |
| Manufacturer Specifications | Available upon request |
| Packing | Export-worthy packing |
| Material Safety Data Sheet | Available on request |
| Product Name | CAS No. |
|---|---|
| Etoricoxib | 202409-33-4 |
| TDP |
|---|
| ✅ |
Send us your Intermediates inquiries to bdteam@sspharma.com
Terms and Conditions: Intellectual Property and Regulatory Compliance
-
Buyer’s Responsibility:
Buyers are responsible for ensuring that the goods they procure and use do not knowingly infringe upon intellectual property rights, including patents, of any party. -
Seller’s Responsibility:
Sellers must refrain from offering goods that violate intellectual property rights, including patents, of any party. -
Research and Development Use Only:
Products protected under active U.S. patents are provided exclusively for research and development purposes, as outlined in 35 USC 271 +A13(1). -
Export of Controlled Substances:
Controlled substances or scheduled drugs are exported solely by the authorized entities of the manufacturer. Such exports require an original import permit from the appropriate regulatory authorities in the receiving country. -
Patent Compliance:
Products covered by active patents will not be offered for sale in regions where such sales would violate patent laws. Buyers are fully responsible for any liabilities resulting from non-compliance with patent regulations. -
Policy on Inquiries:
SS Pharma Group strictly responds only to inquiries submitted with verifiable contact details. Anonymous or pseudonymous inquiries will not be entertained. -
Liability Disclaimer:
SS Pharma Group is not liable for any purchases or usage of products that infringe on intellectual property rights, including patents, of any party. No warranties, whether express or implied, are provided regarding the originality, authenticity, or compliance of products with intellectual property laws.
This website is maintained and regularly updated by SS Pharma Group to ensure transparency and compliance.
